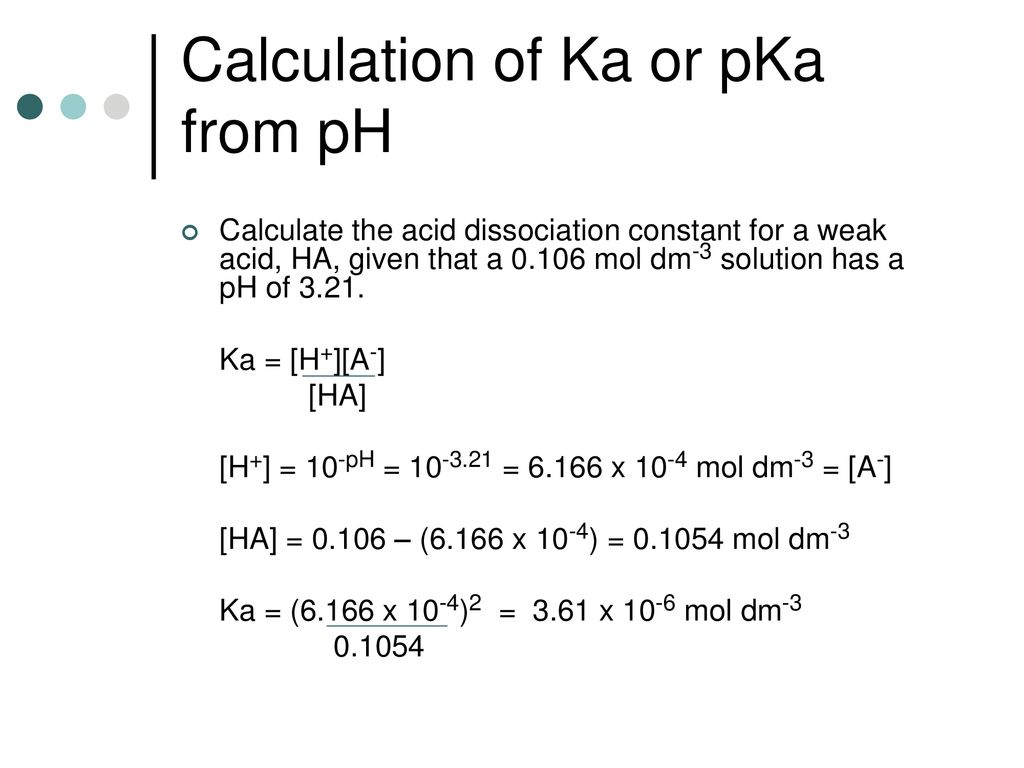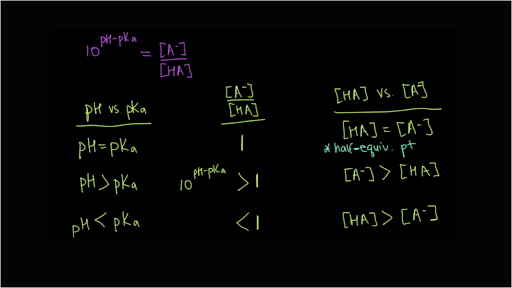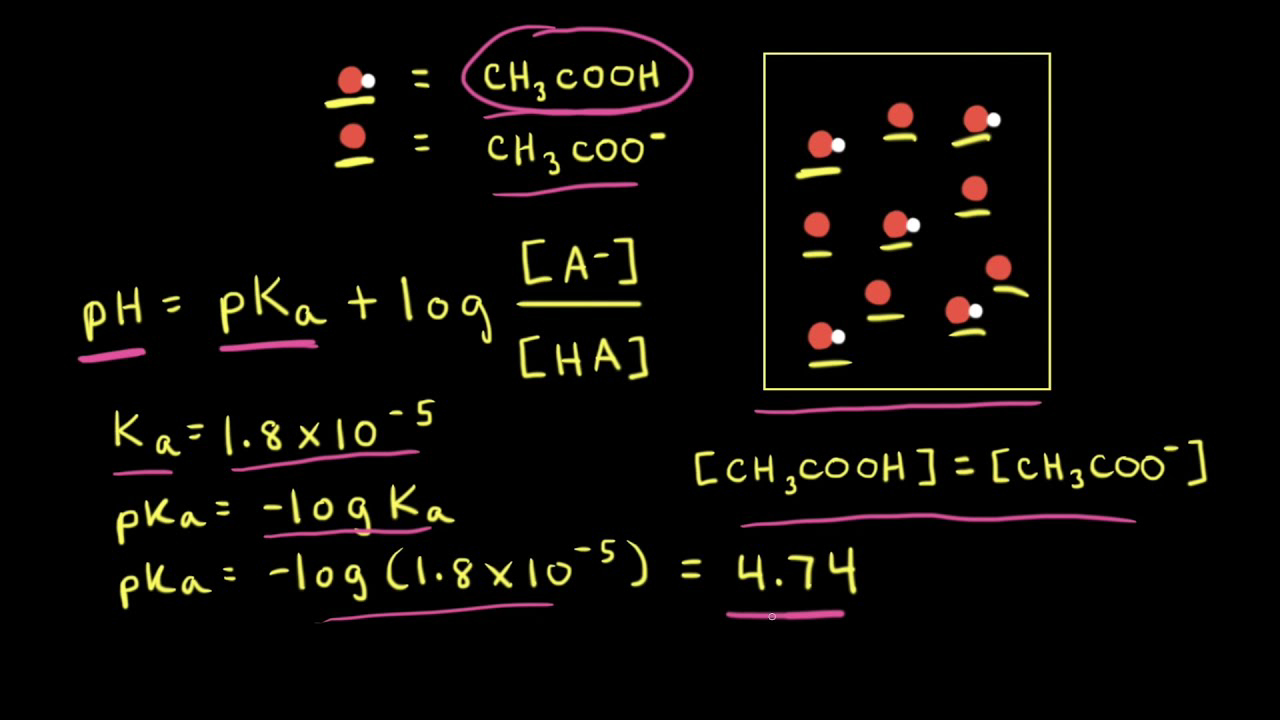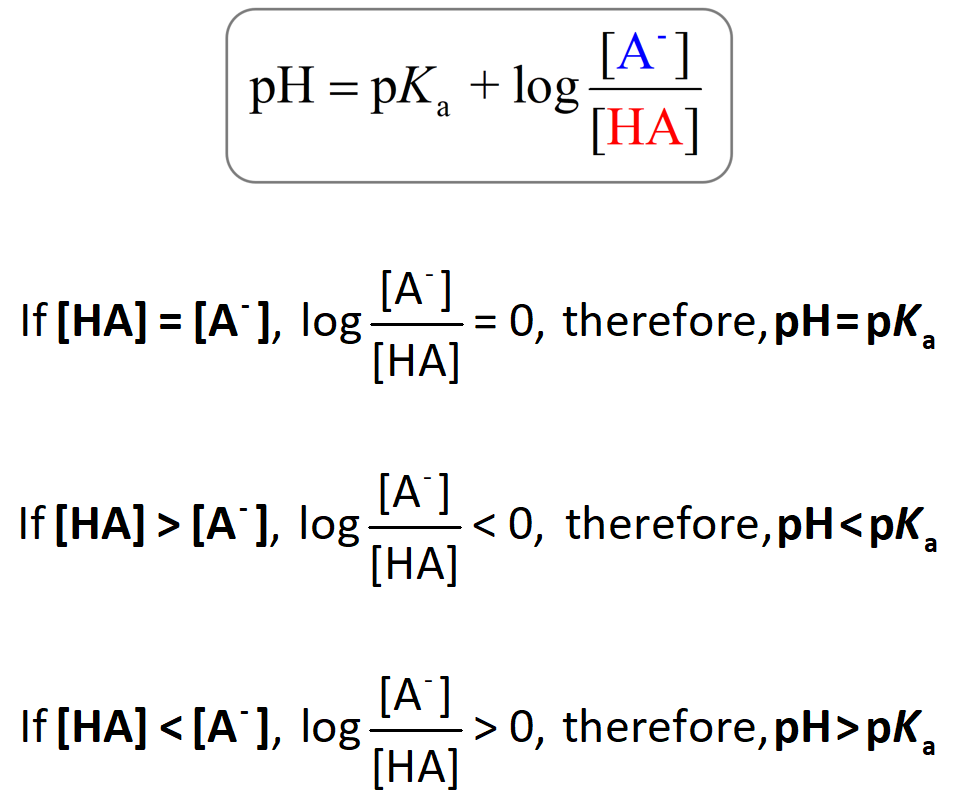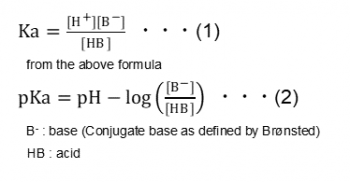
How should the acid dissociation constant pKa be measured? | Automatic Potentiometric Titrators | Faq | Kyoto Electronics Manufacturing Co.,Ltd.("KEM")
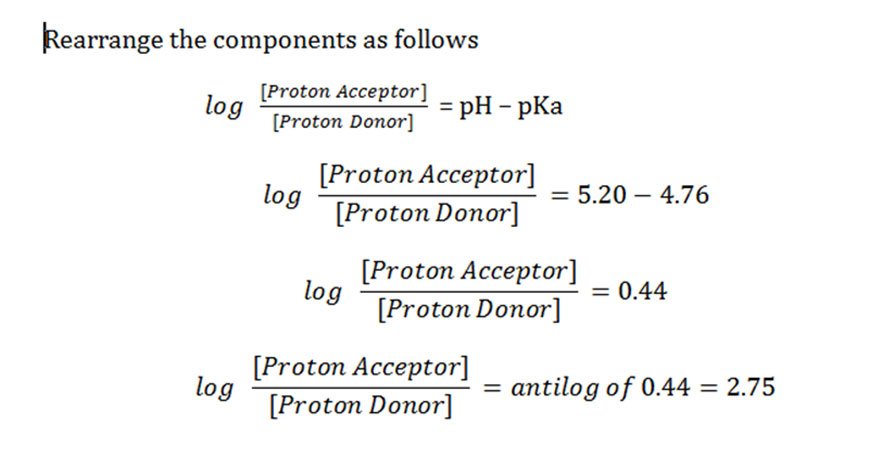
![SOLVED: What is the pH of a buffer composed of 0.10 M oxalic acid and 0.08 M oxalate ion? The pKa for oxalic acid is 1.23. pH = pKa log ([A-J[HA]) Use SOLVED: What is the pH of a buffer composed of 0.10 M oxalic acid and 0.08 M oxalate ion? The pKa for oxalic acid is 1.23. pH = pKa log ([A-J[HA]) Use](https://cdn.numerade.com/ask_images/08a0de4c43b94a8e8f10c82ca0bf2b68.jpg)
SOLVED: What is the pH of a buffer composed of 0.10 M oxalic acid and 0.08 M oxalate ion? The pKa for oxalic acid is 1.23. pH = pKa log ([A-J[HA]) Use

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube

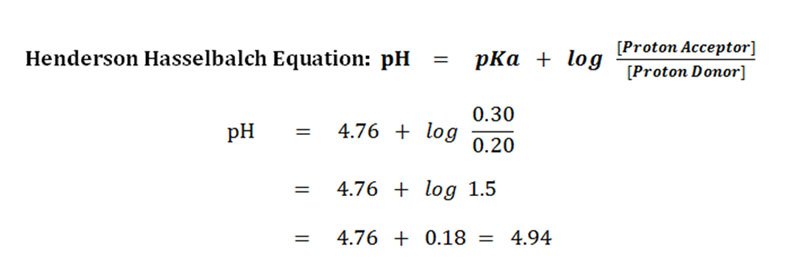
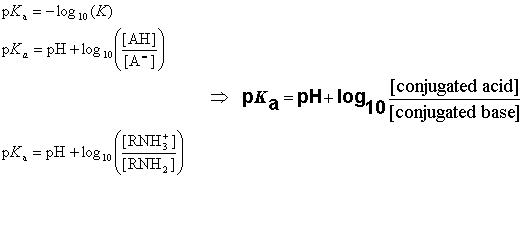
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)