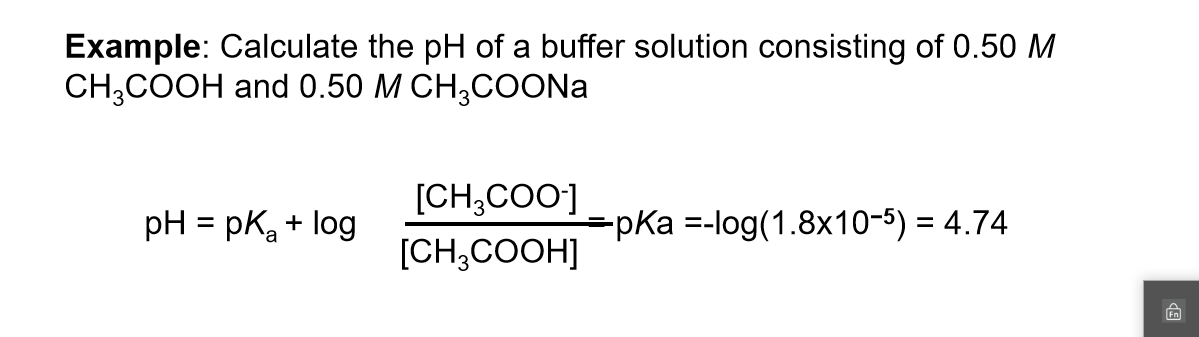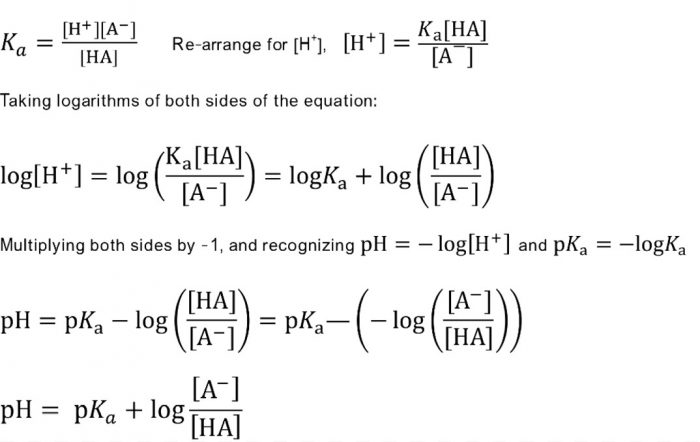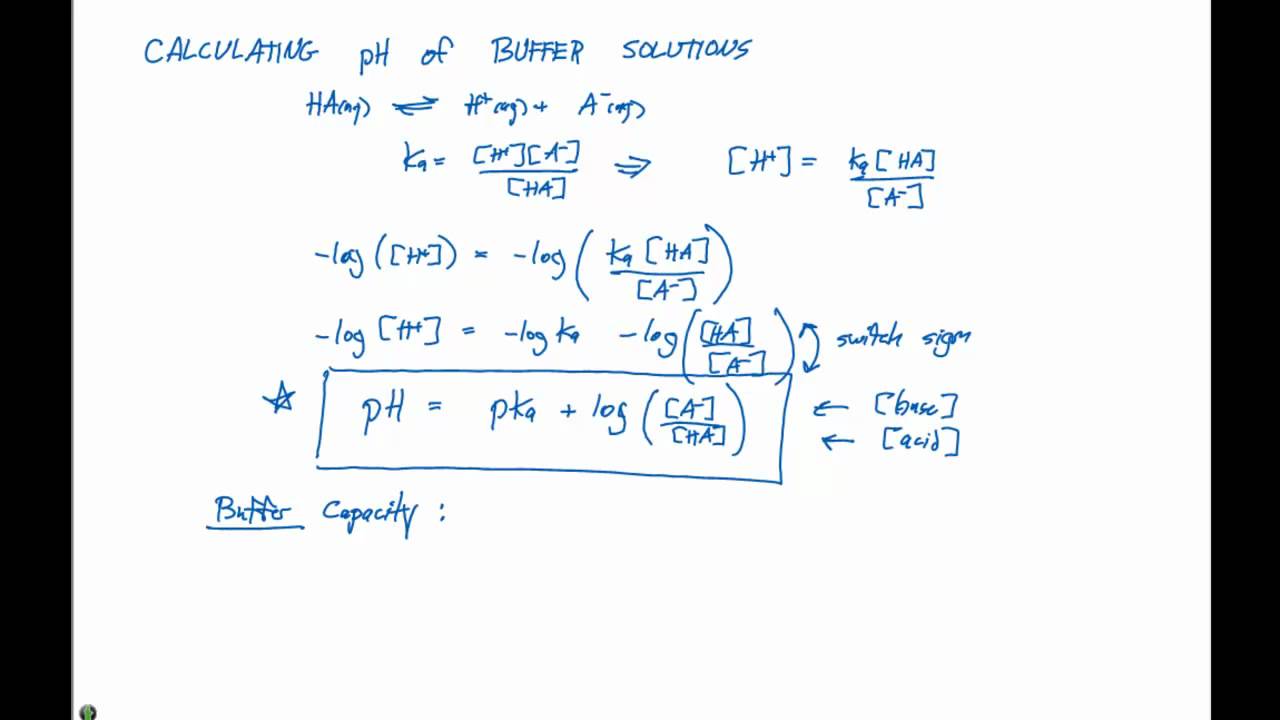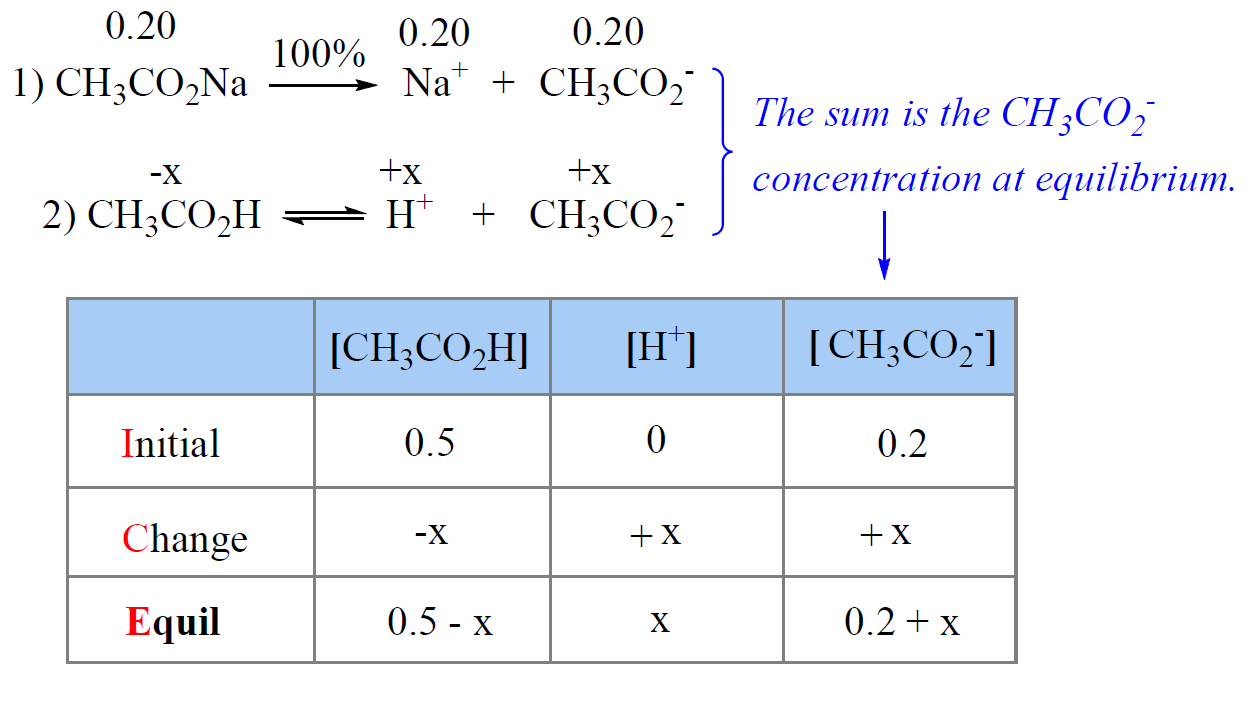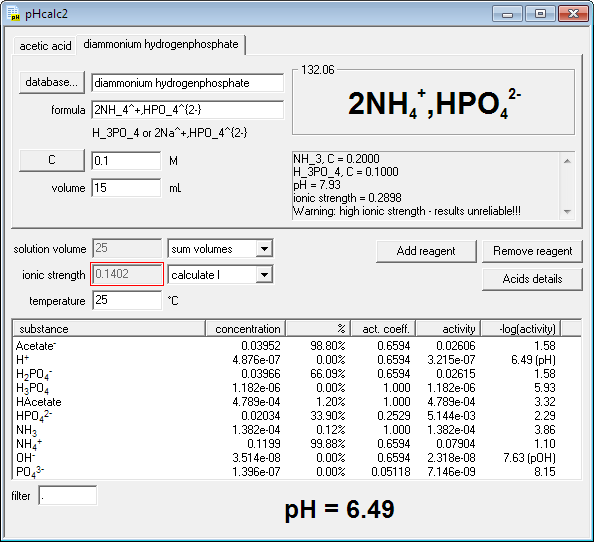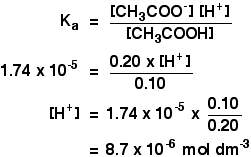
Buffers A buffer is a solution that is highly resistant to changes in pH when a strong acid or base is added. A buffer solution also has a pH close to. -

Calculate the PH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 ml of an aqueous solution containing 150 ml of 1m HCL . ka for HCO^-3 = 5.63 x 10 - 11

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com